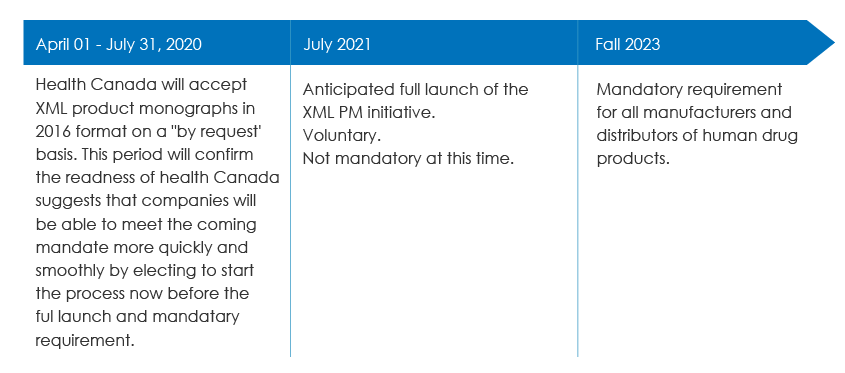
Health Canada accepted HL7 Standard XML product monographs on a “by request” basis from April 1, 2020, to July 31, 2020.
Sponsors can submit a request to file XML product monographs, in the 2016 format, under the following submission types:
- New Drug Submissions (NDS)
- Where a Canadian Reference Product files in the 2016 format, all Abbreviated New Drug Submissions (ANDSs) and Supplements to Abbreviated New Drug Submissions (SANDSs) can also file in the XML format
- Supplements to New Drug Submissions (SNDS), where changes are proposed to SPM format or content of the structured product monograph (SPM)
- All subsequent SPM submissions of a product already filed in the XML format, are also required to continue filing in XML format
This transition period has been established so that both Health Canada and industry systems are in place for the full launch scheduled for Fall 2020. Health Canada plans to make the XML format a mandatory requirement by Fall 2023.
SPM Services
As per the Health Canada mandate, adopting of technology, its processing, managing, labeling and details of any label changes made, including changing the content of the formatted label and changing the carton labeling or container labeling have to be electronically submitted using Structure Product Monogram (SPM) format.
The new Structured Product Monograph is based on Extensible Markup Language (XML), Health Level 7’s (HL7) Structured Product Label (SPL) standard and controlled vocabularies. Based on extensible markup language (XML) the SPM format facilitates the communication of drug labeling data reliability among various groups such as hospitals, prescribing organizations, and doctors, in addition to the general public through Canada Product Database. SPM documents include a header and body. The header includes information about the document such as the type of product and company information and details about its versioning. The body of the document includes product information in both structured with section and subsections in it.
To assist Organizations, Freyr integrates a web-based tool – Freyr SPL-SPM – that automates compliance with current SPM format rules using a unique step SPM conversion methodology for experienced team of conversion specialists, Freyr ensures accurate label conversion and 100% compliance.
Adapting a new change is always a challenge in an industry but this can be overcome with Freyr SPL-SPM integrated tool where it provides a complete solution for Health Canada SPM requirements. Freyr is supporting to perform pilot activities for pharma industry major clients.
Would you like to understand the basic structure of an SPM format? Here we give you a sample SPM submission in English.
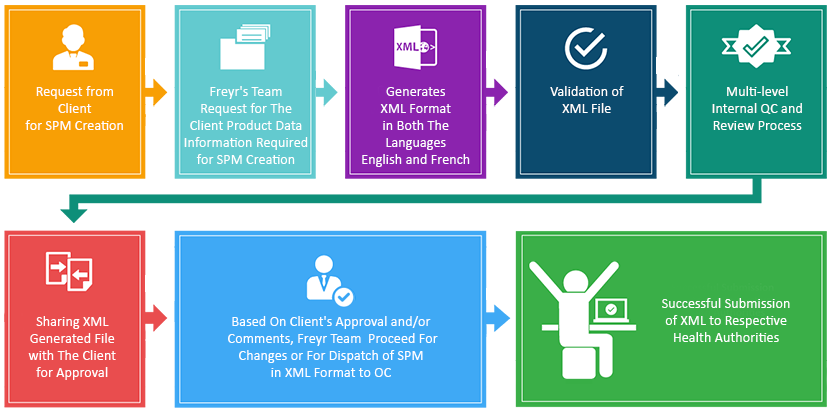
SPM Submission Process
SPM Submission Timelines
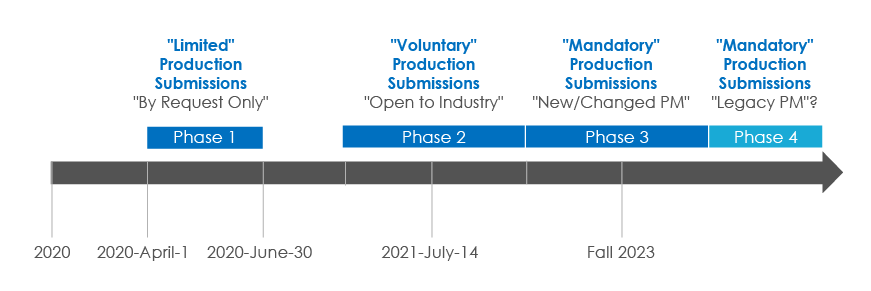
SPM Submission Phases
With all the timelines and phases explained, now is the time for compliant transition. Would you like to understand how different is SPM from Structured Product Labeling (SPL)? Or do you wish to know more about Freyr’s SPM roadmap?
