SPM Readiness
Health Canada started accepting HL7 Standard XML Product Monographs on a “By Request” basis.
The actual deadline for the transition was ended on July 31, 2020. The transition period was established to enable the industry to be in place for the full launch scheduled for Fall 2020. Health Canada is currently in plan to make the XML Product Monograph (PM) format a mandatory requirement by Fall 2023.
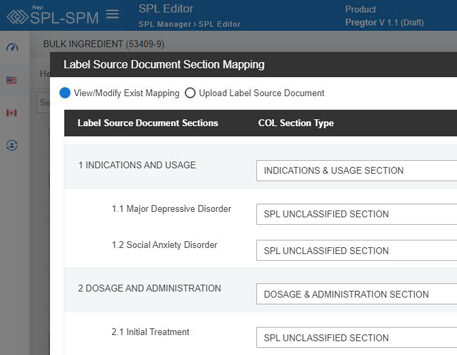
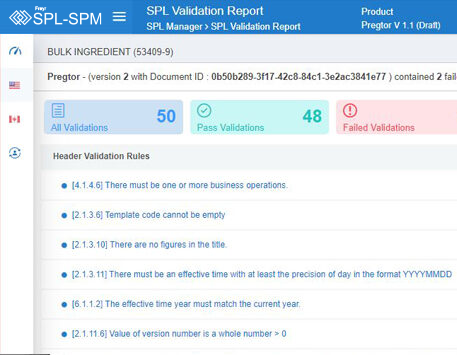
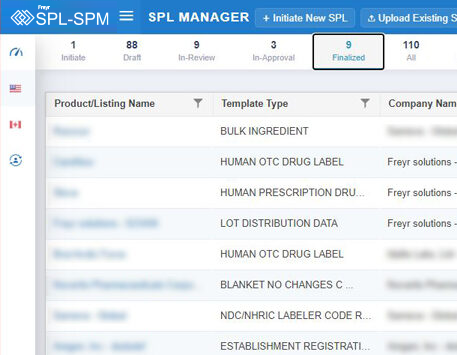
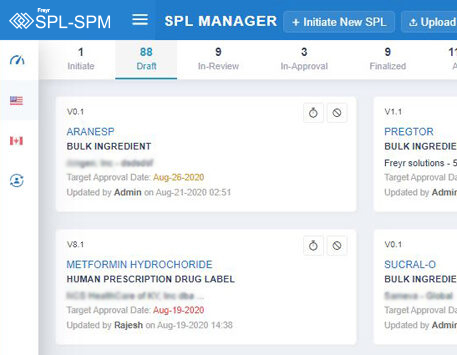
Decode More About XML Product Monographs or SPM.
KNOW MORE